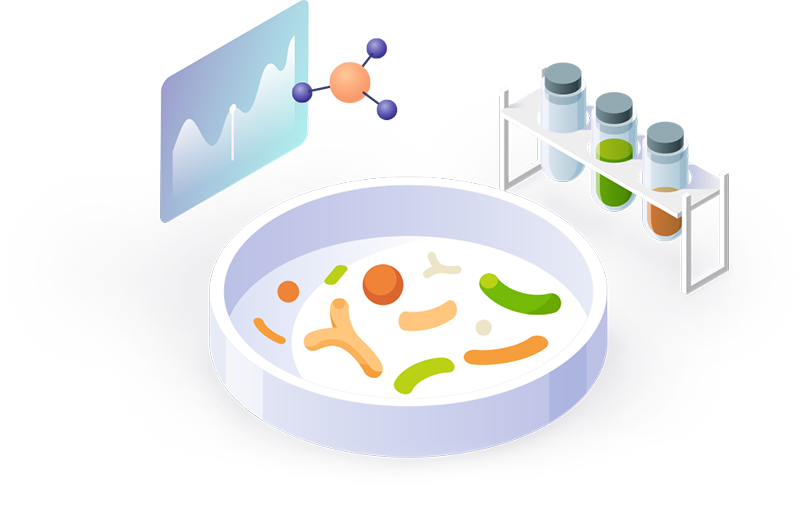
Well Documented StrainsNumerous Scientific Studies Prove Safety of Strains
A total of 83 published papers, including 26 clinical papers and 7 genome sequencing papers, have confirmed the safety and efficacy of DUOLAC probiotic strains.
Precise Internal Tests
The safety of the microorganism begins with identification.
DUOLAC strains are identified through proven state-of-the-art technology.
-
Finding Special Genetic Information from the Full Genome Sequence
Through full genome sequencing, we predict the function of each strain using cutting edge NGS technology.
-
16S rRNA Sequence Analysis
This is an accurate strain identification process using genetic analysis technology.
-
Antibiotic Resistance Test
The test conducted fully satisfies European Food Safety Authority standards (EFSA criteria).
-
Toxicity Assessment
All DUOLAC strains are tested and confirmed as non-toxic and safe for commercialization.
-
Physiological Function Test
Animal testing is used to identify various physiological functions of the strains.
Strain Deposit
at Certified Institutions
To ensure the safety of commercial probiotic strains, each strain is deposited in domestic and overseas microorganism collections, namely KCTC, DSMZ and NCBI.
All DUOLAC probiotics strains receive a unique strain number and are securely stored and managed in authorized institutions in South Korea and Germany.
-

Strain registered to Korean Collection for Type Cultures (KCTC)
-

Strain registered to Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ)
-

DNA Sequence registered to the U.S. National Center for Biotechnology Information (NCBI)